Before i can tell you how the Haber Bosch process feeds the world , Let me explain what is the Haber Bosch process.
This process which won 2 scientist nobel prizes in 1918 and 1932 . Also known as the Haber process , the Haber-Bosch process is used for the manufacturing of ammonia.
N2 (g)+ 3 H2 (g) ⇌ 2 NH3 (g)
The reactants are nitrogen and hydrogen.
-Hydrogen( H2) is obtained from reacting natural gas (usually methane) with steam or cracking hydrocarbons
Methane + Steam (water) -> Carbon dioxide and Hydrogen
-Nitrogen ( N2) is obtained from air as air is nearly 80% nitrogen and 20% oxygen. The oxygen is removed by burning hydrogen
Hydrogen + Oxygen -> Water
The Product is ammonia (NH3 )
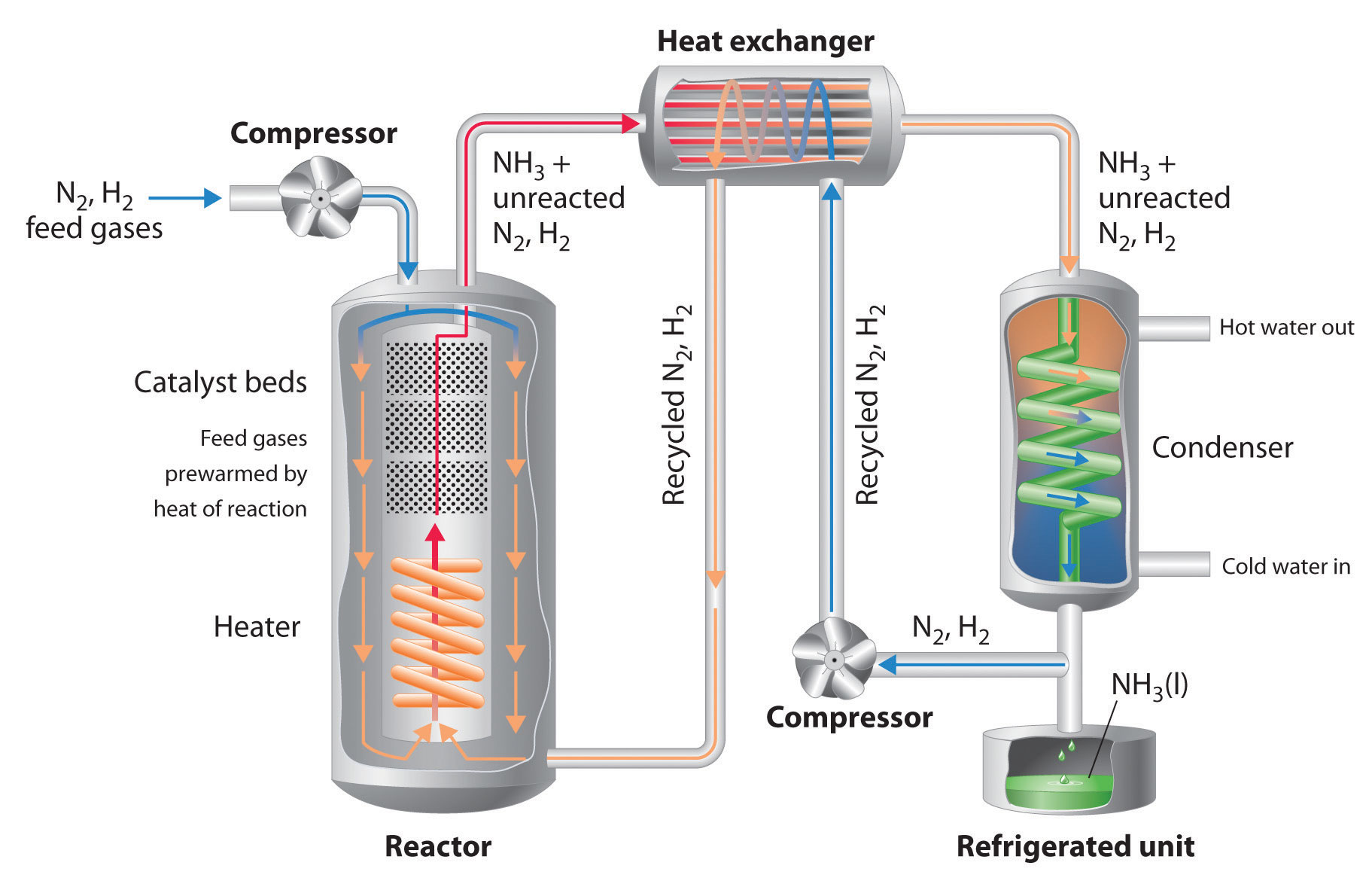
Brief diagram of the Haber-Bosch process - http://newsletter.flatworldknowledge.com/bookhub/reader/4309?e=averill_1.0-ch15_s06
Relation with equilibrium
_______________________
This reaction is a reversible reaction and unreacted reactants are re-used hence we show this with the equilibrium sign ⇌


